Research
Clinical Trials UnitThe Clinical Trials Unit (CTU) of Northwestern facilitates innovative clinical cardiovascular research to advance scientific knowledge, prevent disease and improve health care for all patients. Our research efforts include multicenter clinical trials on medical therapy, minimally invasive technology, device implantation and surgical options.
The CTU is affiliated with Northwestern Memorial HealthCare and Northwestern University Feinberg School of Medicine. These affiliations strengthen our ability to conduct meaningful clinical research and to support our clinical investigators. The CTU staff ensures compliance with federal, state and institutional regulations to protect the safety of our research participants. |
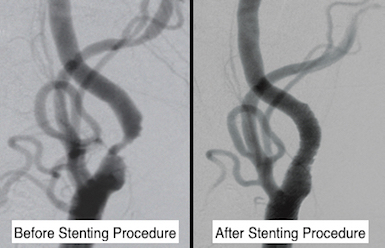
CREST-2
The carotid arteries are major blood vessels in the neck that supply blood to the brain, neck and face. CREST-2 is a clinical trial for people who have narrowing of their carotid artery, without stroke warning signs such as mini-strokes. CREST-2 is designed to compare three different methods of stroke prevention to find the safest and most effective treatment: intensive medical management alone or in combination with procedures to reopen the carotid artery (carotid endarterectomy or carotid stenting).
“The drugs that society has available today are much more advanced than the last time medical therapy was studied in CREST-1. The goal of CREST-2 and the reason Bluhm Cardiovascular Institute is involved, is to evaluate the effectiveness of current medical therapy for patients at risk of stroke due to carotid artery disease,” says Mark K. Eskandari, MD. |
Surgical Options For Advanced Heart Failure
Patients with advanced heart failure may benefit from ventricular assist devices (VADs) at various stages throughout their disease progression. VADs are primarily used as either a bridge to heart transplant to keep patients alive while they wait to receive a donor heart or as destination therapy to provide a permanent alternative in patients who are not heart transplantation candidates.
“We have programs in place to treat both acute and chronic heart failure patients. Temporary support devices are one of the options used to stabilize patients in an acute state of heart failure, while VAD and heart transplant address long term needs,” says Duc Thinh Pham, MD. "Pioneering the latest technology at Bluhm Cardiovascular Institute enables us to have multiple treatment options for all our heart failure patients." |
Regenerative Medicine
|
Cardiovascular Imaging Research
|
Atrial Fibrillation: Reducing Stroke Risk
Pulmonary Vein Isolation Ablation (PVI) is a procedure used to treat atrial fibrillation (AF). This type of ablation works by scarring or destroying tissue around the pulmonary veins that may trigger AF.
The left atrial appendage (LAA) is a pocket-like section in the left atrium (upper chamber) of the heart that is often a source of blood clot formation that may cause up to 90 percent of strokes due to AF. The LARIAT procedure sutures shut the LAA from the outside of the heart potentially reducing risk of blood clots and stroke due to AF. Bluhm Cardiovascular Institute is recruiting patients with persistent or longstanding persistent AF into the aMAZE Study: LAA Ligation Adjunctive to PVI for Persistent or Longstanding Persistent Atrial Fibrillation. “aMAZE is a clinical trial designed to compare the safety and efficacy of two different methods of AF treatment: PVI alone or PVI with the LARIAT procedure,” says Albert C. Lin, MD. |
Ongoing Clinical Trials List
The Clinical Trials Unit (CTU) of Northwestern is currently conducting clinical research in the following specialty areas. The CTU was established in 2005 to provide the infrastructure necessary to assist investigators in performing high-quality clinical research trials.
Coronary Disease Heart Failure Heart Rhythm Disorders Heart Valve Disease Preventive Cardiology Vascular Disease |
|
© 2024 by Northwestern Medicine® and Northwestern Memorial HealthCare. Northwestern Medicine® is a trademark of Northwestern Memorial HealthCare, used by Northwestern University.
|